From Final Report of the International MOX Assessment, COMPREHENSIVE SOCIAL IMPACT ASSESSMENT OF MOX USE IN LIGHT WATER REACTORS, J. Takagi, M. Schneider, F. Barnaby, 1. Hokimoto, K. Hosokawa, C. Kamisawa, B. Nishio, A. Rossnagel, M. Sailer, IMA Project, Citizens' Nuclear Information Center, November 1997
Safety Aspects of Unirradiated MOX Fuel Transport
Edwin S. Lyman
Introduction
As a result of several factors, it is becoming increasingly likely that in the future, large quantities of commercial plutonium will be shipped internationally not in the form of oxide powder, as was the case during the controversial sea shipments of 1984 and 1992, but in the form of finished mixed-oxide (MOX) fuel assemblies. These factors include: (1) the delay or cancellation of MOX fuel fabrication facilities in the nations which are foreign customers of reprocessing companies, such as Germany and Japan; (2) the expansion of MOX fabrication capacity in the countries that provide reprocessing services; (3) the reprocessing industry's hope that the transport of plutonium in the form of MOX fuel will be perceived by the public as posing less severe environmental and security risks than the transport of plutonium oxide, and will therefore generate less controversy.
Will transport of unirradiated MOX fuel (U-MOX), as currently conceived, be less dangerous than transport of plutonium oxide? In general, the answer is yes -- the risk that a large fraction of the contents of a shipping package will be dispersed as a result of a transport accident is smaller if the material is shipped in consolidated form rather than as a powder, if all other aspects of the transport are identical. However, if one considers the actual packaging systems for transport of U-MOX that are now in use or will be used in the future, it is apparent that U-MOX package designs tend to exploit the less dispersible nature of the contents through omission of one of the multiple containment barriers that would be present in a plutonium oxide transport cask. For example, US domestic regulations require that plutonium in dispersible forms be shipped (by land) in packages with two independent containment barriers, but shipping packages for "nondispersible" forms, including U-MOX, need only have one.
In this article, I will argue that the current regulations governing transport of U-MOX provide inadequate assurance that the risks of such transports will be acceptably low. The recently approved 1996 revision of the IAEA's Safety Series No. 6 (SS6), which promulgates international standards for the shipment of radioactive materials (RAM), was carefully constructed to legitimate the risky practices now utilized for transport of U-MOX. These standards are not intended to provide protection in the event of very severe transport accidents, which could result in a widespread dispersal of plutonium from a cargo of U-MOX. The revised IAEA regulations will do little to alleviate the serious safety concerns associated with sea and air shipment of radioactive materials in general, and U-MOX in particular.
Type B Package Standards and the Notion of "Graceful Failure"
305
Under the 1985 (revised 1990) edition of SS6, which will remain in effect until the 1996 version is adopted by individual IAEA member states (a process that can take years), unlimited quantities of plutonium in any form can be transported by land, sea or air in so-called "Type B" casks. Type B casks are designed so that they will lose only a fraction of their contents (a radionuclide-specific quantity known as "A2" per week, an amount deemed "acceptable" by the IAEA and which is tabulated in SS6) if they are exposed to accident conditions equivalent to an impact of 13.2 meters per second (m/s) on an unyielding surface, followed by an fire with a flame temperature of 800 degrees C for 30 minutes.
It has been pointed out by many observers on many occasions that the conditions that a RAM package may encounter in the course of an accident at sea or the crash of an aircraft can be far more severe than those simulated by the Type B test. One of the chief arguments invoked by the IAEA in response is the notion of "graceful failure": namely, the claim that RAM packages are designed and constructed with such a high degree of conservatism that they will be able to withstand accident conditions far more severe than those under which they are tested.
The "graceful failure" principle is central to the philosophy on which the 1996 version of SS is based, as will be discussed further below. However, there is hardly any experimental evidence for it, as the IAEA freely admits. [1] Package manufacturers have little incentive to carry out testing to a severity beyond what the standards require; such tests are expensive and difficult, and they present the risk of embarrassment should the package fail abruptly rather than gracefully. [2] In the absence of experimental verification, "graceful failure" is based entirely on expectation.
While the graceful failure concept may have limited validity in the case of spent fuel casks, for which the shielding requirements mandate very thick (25 cm) metal walls that also provide mechanical strength and thermal insulation, the Type B packages used for transport of U-MOX require far less shielding. A review of available information on U-MOX transport cask designs does not provide confidence that they can withstand mechanical and thermal loads far in excess of those for which they were designed.
A typical Type B U-MOX fuel cask design is the Westinghouse Model No. MO-1. Although the 1976 NRC license for this design was allowed to lapse, it is considered representative of the type of package that will be used in the US for the transport of U-MOX, should the US carry out its plan to dispose of some excess weapon plutonium by fabricating it into MOX and irradiating it in reactors. [3] The MO-1 is designed to transport 2 MOX assemblies containing about 45 kg of
306
plutonium. The package weighs 3.9 tonnes and has outer dimensions 1.143 m x 1.194 m x 5.23 m. It consists of two concentric steel shells, each around 3 mm thick. The volume between the shells is filled with an 18-cm thick layer of rigid polyurethane foam to provide shock and thermal insulation. The MOX assemblies are separated by borated stainless steel plates for criticality control. Because of the importance of insulating the assemblies from shocks during routine transport, they are shock-mounted inside the package with clamps.
Less information is publicly available about the Transnucleaire FS-69, the Type B package currently used in France to transport U-MOX assemblies by road, and presumably the same one that will be used for international shipments. The FS-69 is a Type B package licensed for the transport of U-MOX containing plutonium with up to 30,000 parts per million (approximately 3 weight-percent) americium-241 (Am-241). This corresponds to plutonium obtained from spent fuel-of 45,000 MWD/t burnup and aged for six years, which is the plutonium composition used in the design of the MELOX plant.
Details of the FS-47 are considered proprietary. However, from the general descriptions that can be found in the open literature, the cask can be seen to have many similarities to the MO-1: it carries two assemblies held in place by a borated aluminum basket, weighs 5 tonnes when loaded and consists of two concentric steel shells separated by a neutron-absorbing material. [4] On the basis of the weight similarity with the MO-1, the FS-69 must contain a comparable amount of structural metal.
It is reasonable to assume that with respect to the construction materials and overall design philosophy, the FS-69 is similar to the FS-47, the container that was used to transport cans of plutonium oxide by sea in 1993. The FS-47 structure, like that of the MO-1, is based on two thin concentric steel shells (outer shell a few millimeters thick; inner shell about 1 cm thick) separated by a 5-cm thick layer of heat insulation ("wet", or hydrated, gypsum) and a 15-cm thick layer of a (proprietary) neutron absorber material. [5]
No information on the type of seal used in the FS-69 could be located. The ability of a cask to maintain containment if exposed to a beyond-design-basis fire depends crucially on the seal material. For instance, the elastomer seals used in the Transnucleaire TN 28 VT cask for vitrified high-level radioactive waste (VHLW) will fail if they are heated to a temperature above about 250 degrees C, which could occur if the cask were exposed to an 800 degrees C fire for a couple of hours. According to COGEMA, the seal of the FS-47 cask failed after it was exposed to a 1000 degrees C fire for 1.5 hours; [6] if the FS-69 uses the same type of seal it can be expected to behave similarly.
307
Even less information is available about the "mystery" package now used by BNFL to transport MOX by air to Switzerland. A recent request for details about this cask was denied by BNFL, on the grounds that "disclosure of package types and capabilities would result in a breach of customer confidentiality." [7] However, according to BNFL, "... all packages for MOX transport fulfil the requirements of all the appropriate transport regulations." All this means at present is that the package used to ship MOX by air is Type B, the significance of which will be discussed below. However, one would expect that the package would not be more robust than the FS-69 used by BNFL's commercial competitor, Cogema. BNFL had attempted in the past to design a package specifically for air transport of plutonium oxide, known as the 1680. However, "due to changing commercial priorities ... it has not yet been used." [8]
It, is apparent from what little is publicly known of these package designs that their structural strength and thermal resistance is provided primarily by the filler material between the two steel shells, which are themselves too thin to provide much strength. However, materials such as rigid polyurethane foam typically are not capable of providing resistance to mechanical or thermal stresses well in excess of design stresses. If the energy of an impact is significantly higher than the design energy, the foam will be crushed without causing any deceleration of the package. However, increasing the resistance of the package to high energy impacts by using a denser foam would increase the risk that the package could not withstand lower energy impacts, since the foam will not compress (and therefore act as a rigid surface) if the energy is too low. [9] In other words, for any foam density, there is a fairly narrow "window" of impact energies for which the foam is capable of providing protection.
"Wet" (hydrated) gypsum is a material used in boards for building construction. It is not capable of load-bearing and therefore cannot provide the FS-47 cask with significant impact resistance.
With regard to thermal resistance, organic materials such as polyurethane foams function primarily via ablation - that is, they absorb heat energy by burning and carry heat away from the payload by dispersal of combustion products. Therefore, they can only continue to protect against a fire until they are completely consumed. After this point, the contents of the cask will quickly achieve thermal equilibrium with the fire temperature. In thermal tests of a polyurethane foam shielded by a metal lid, more than 25% had degraded after exposure to a flame of approximately 980 degrees C for fifteen minutes. [10] Thus the "graceful failure" margin for casks that rely on ablative media for thermal protection is rather slim.
308
In the FS-47 cask, thermal protection is provided by the layer of hydrated gypsum, which has a low thermal conductivity at temperatures around 40 degrees C. However, hydrated gypsum can decompose at temperatures as low as 150 degrees C, and it cannot be expected to provide a significant margin of safety against fires of greater severity than the design basis fire.
Behavior of Unirradiated MOX Fuel Under Accident Conditions
Thermal: Although U-MOX is a refractory material with a very high melting point (around 2700 degrees C), if it is heated in the presence of oxygen it readily oxidizes, expands and undergoes comminution (production of fine particles). This process can take place at temperatures as low as 250 degrees C, so that significant oxidation and comminution is possible even in thermal conditions of moderate severity. [11] In particular, prolonged thermal exposure at relatively low temperature, as is characteristic of smoldering conditions following a severe fire, could result in substantial particulate formation.
Oxidation can only occur, by definition, if the fuel pellets themselves are exposed to oxygen. Therefore, for oxidation to occur mechanisms must exist for failure of the cladding of fuel rods and for the cask seal (if the casks are filled with inert gas; otherwise, oxygen will be present in the cask even if the seals initially remain intact). Fuel rod cladding can be ruptured either by mechanical impact during the accident or by bursting as a result of thermally induced overpressure. The latter has been observed to occur in spent fuel rods after exposure to temperatures over 725 degrees C for a four-hour period. [12] Extrapolation of this result to unirradiated fuel rods is not straightforward, because they contain no fission gas and their cladding has not undergone wastage from interaction with fuel and coolant during reactor operation. However, the former will not make much difference for PWR fuel rods, since they are pressurized with helium fill gas, and fission gas would only increase the internal pressure by a few percent.
If the cladding is breached due to mechanical impact, oxidation of the fuel will cause it to expand and exert additional pressure on the cladding, which will cause further ruptures and exposure of the fuel pellets to air.
If oxygen access to the fuel pellet surfaces is limited, then there will be little particulate formation. However, the volatility of americium-241 in the fuel will be greatly enhanced in reducing conditions, which could result in the release of highly radiotoxic americium vapor from the fuel during a fire, even if the fuel matrix itself is not dispersed. Transport safety literature has paid no attention to this phenomenon. In contrast, volatilization of plutonium (e.g. gaseous release) is not likely to occur to a significant extent for the range of temperatures that would be encountered in a transport accident.
309
Mechanical: Uranium oxide ceramic pellets are brittle materials, and shatter when exposed to high-energy impacts. The size distribution of particles produced by such impacts is typically log-normal. For example, experiments on depleted uranium pellets subjected to an impact of energy 0.1 J/g, corresponding to an impact on an unyielding surface of 14.1 m/s (slightly higher than the IAEA Type B impact speed) have found that the pellets will release approximately 1% of their mass as particles with diameters less than 100 microns (called the "dispersible fraction"), and 0.01% as particles with diameters less than 10 microns (called the "respirable fraction") [Fig 1]. [13]
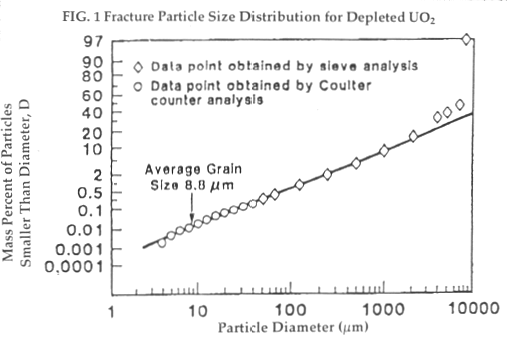
Higher impact speeds shift the distribution in the direction of smaller average particle size [Fig 2], and thus in the direction of increasing hazard. [14] (The "dispersible fraction" denotes particles that can be transported easily in the form of aerosols; larger ones tend to settle rapidly. The "respirable fraction" denotes particles that tend to remain deep in the lung once inhaled.)
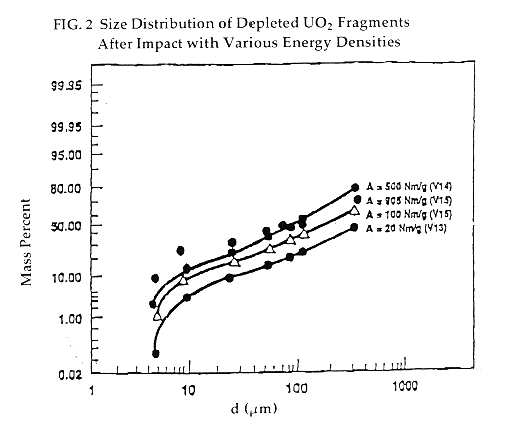
One must rely on uranium oxide impact data because there is little or no information available on actual MOX pellets. However, differences in the microstructure of the two fuel materials may affect the impact behavior.
Transport of U-MOX by Sea
There are numerous historical examples of shipboard fires of much greater duration than that represented by the SS 6 Type B test. For instance, some fires have burned for days or even weeks. It has also been noted that the combustion of hydrocarbon fuels can result in considerably higher maximum temperatures --- as high as 1300 degrees C --- than are simulated by the 800 degrees C test. [15]
After enduring years of criticism by outside experts, IAEA recently initiated a Coordinated Research Program (CRP) to analyze whether the Type B test indeed provides adequate protection for RAM transport by sea. However, it is questionable whether the CRP will provide an objective evaluation of the situation, because its underlying premise is not to question, but rather to confirm, the adequacy of the IAEA standards, according to one of the participants in the project. [16]
It is worth noting that the international marine transport of RAM is essentially an unregulated practice. RAM transport was intentionally excluded (as a result of IAEA intervention) from the Safety of Life at Sea (SOLAS) convention, which is a binding international
310
agreement mandating design specifications for ships carrying dangerous goods. The "Irradiated Nuclear Fuel" (INF) Code, which was adopted by the International Maritime Organization (IMO) In an attempt to narrow this RAM loophole, is a voluntary agreement only. Even under this nonbinding code, it is acceptable to transport as much as about one tonne of reactor-grade plutonium (e.g. about 40 U-MOX assemblies) on non-purpose-built passenger (INF 2 class) vessels.
In the event that a MOX transport vessel experiences an accident of greater severity than a Type B package is designed to withstand, the amount of material released will be determined by the "graceful failure" behavior of the package. As discussed above, U-MOX packages that use ablative materials for fire protection will not be able to withstand a prolonged fire (greater than a few hours). Of special concern is an accident which first causes the rupture of many fuel rods and is then followed by a long-duration fire. Even if the fire smolders at a low temperature, substantial oxidation of the fuel rods can take place if the package is ruptured or the seals fail. The amount of fuel oxidized would be limited only by the duration of the fire and the availability of oxygen.
Transport of U-MOX by Air The Low Dispersible Material (LDM) Exemption
An alternative to sea shipment of U-MOX fuel from Europe to Japan is air shipment. Air shipment of plutonium and other radioactive materials has been a controversial practice, largely because of the inadequacy of the IAEA air transport standards. Past versions of SS6 were essentially "mode-independent": the same package standards (Type B) applied for both ground and air transport, despite the fact that the mechanical and thermal stresses encountered in a plane crash would be far greater than those simulated by the Type B test. This deficiency was highlighted most forcefully by the United States, which unilaterally adopted very stringent domestic regulations on air transport of RAM.
In response to public criticism and the disparity between SS6 and U.S. domestic regulations, the IAEA made a feeble attempt to increase the credibility of its standards. The 1996 revision of SS6 defines requirements for packages, called "Type C," which are intended for the air transport of large quantities of RAM. The Type C test includes an impact of 90 m/ s on an unyielding surface, and a non-sequential 800 degrees C, 1-hour fire. Although the Type C standards appear to be more rigorous than the Type B standards, they fall far short of the US domestic regulations, and in fact were intentionally chosen so that Type C casks would only survive 85-90% of air crashes (the so-called "knee of the curve" point beyond which significant improvements would come at too great a cost to industry, in the IAEA's judgement).
Having introduced a new but still inadequate standard for air shipment of RAM, IAEA then proceeded to include an exemption from the Type C requirement for so-called "low dispersible material" (LDM). While the actual text makes no mention of specific materials which may fit the LDM criteria, it is widely understood that the primary beneficiary of this exemption will be transporters of U-MOX assemblies, who would be free to transport unlimited quantities of U-MOX in Type B casks should it be demonstrated that U-MOX meets the LDM criteria.
311
One strong indication that plutonium shippers fully expect that they will be able to transport U-MOX by air in Type B casks is the noticeable lack of development work in recent years by the nuclear transport industry on the Type C cask, even though introduction of the regulation was anticipated as long as a decade ago. For instance, at the 1995 PATRAM (Packaging and Transport of Radioactive Materials) Conference, there was not a single paper on RAM air transport packaging, in stark contrast to the 1989 and 1992 meetings, at which there were entire panels on the subject. One suspects that attempts to develop Type C packaging for plutonium were abandoned once it became clear that an exemption applicable to U-MOX would be included in the revised regulations.
This has been confirmed in a recent letter from BNFL, which stated that "BNFL has undertaken some provisional tests to establish whether MOX fuel might be classified as Low Dispersible Material. The results of these test [sic] were encouraging…" [17] BNFL gives no further details or references to support their claim.
The certification process for LDM is as follows: the test material will be subjected to the Type C impact and fire tests (also non-sequentially), without the protection of any packaging. The material qualifies as LDM if it then does not release an amount of activity greater than 100 A2 in gaseous and particulate forms of up to 100 microns in diameter. As is the case with package tests, compliance can be shown by "direct physical tests, analytical methods or a proper combination of these."
This precision of this definition, which was originally introduced by Germany, falsely gives the impression that it was derived from detailed technical analysis. In fact, a review of the supporting documents shows that the technical basis of the LDM definition is obscure. The radiological arguments advanced in the German working papers on the subject seem crude and arbitrary. [18] There is some evidence, however, that the definition may have been constructed from German data on the oxidation behavior of MOX fuel pellets so that U-MOX would qualify automatically.
The first odd aspect of the LDM definition is that it appears to be inconsistent with the radiation protection standards upon which the remainder of the SS6 is based. For instance, following an accident, a Type B or Type C package is supposed to release an amount of activity no greater than A2 in one week (with no restriction on particle size). However, material qualified as LDM can release more than one hundred times that amount (100 A2 in particles with diameters less than 100 microns, plus an unlimited amount of activity in particles greater than 100 microns). Therefore, in order for the transport of LDM to be consistent with this standard, the Type B package must be in sufficiently good shape following a plane crash that it can prevent more than 99% of the activity released from the LDM from escaping into the environment. This invocation of the "graceful failure" hypothesis requires that one believe that a Type B cask will remain largely
312
intact if it is subjected to an impact energy 50-100 times greater than that which it was designed to withstand.
The German working group papers on LDM are vague on exactly why they believe their criterion provides an acceptable standard. At one point, they make reference to "graceful failure"; at another, they cite the low rate of aircraft accidents; at another, they cite the effect of atmospheric dilution of the release. None of these explanations provides a convincing rationale for adopting release rates for LDM that are inconsistent with Type B and Type C release rates.
The definition of the LDM test proposed by Germany also underwent a significant change. Originally, the German proposal suggested that the impact and fire tests be conducted sequentially, because they realized that the fire test alone conducted on a sample such as a MOX fuel rod would probably cause little or no dispersal, whereas a fire following an impact which ruptured the fuel rod would cause a much greater release; thus, use of a non-sequential test would give a misleading impression of the non-dispersibility of the material. However, they proposed that the fire test in the sequence be limited to only 10 minutes at 800 degrees C. [19] In later versions of their proposal, the recommendation that the impact and fire tests be performed sequentially was omitted.
A 1982 German paper on the oxidation behavior of MOX fuel pellets in a kerosene fire may shed some light on the mysterious origins of the LDM standard. [20] In this paper, it was found that exposing MOX pellets to an 800 degrees C fire for 15 minutes in an air atmosphere led to a release of 0.01% of the initial material. The particles formed were all well below 100 microns in diameter. Therefore, for a Type B cask carrying about 45 kg of plutonium in two MOX assemblies, this release fraction would correspond to a release of 4.5 grams of plutonium, which is very close to 100 A2 = 5 grams (for a typical reactor-grade plutonium composition). Thus it is possible that a release of 100 A2 was proposed by Germany based on their expectation of how a MOX fuel rod would perform with respect to the initial LDM test proposal (e.g. an impact test, which would cause the fuel pellets to be exposed but might not itself lead to a significant release, followed by a 10-minute fire test). Even though the test specifications later changed, the original LDM definition was retained, probably because of the initial observation that the fire test alone would probably not lead to release of any material from a MOX fuel rod, even for a duration of one hour.
Despite this apparent effort to work backward from the known properties of MOX fuel to devise a definition of LDM, it remains highly uncertain whether U-MOX will be able to meet the LDM standard, especially with regard to its impact resistance. An impact of 90 m/s corresponds to an energy input of around 4 J/g, which, based on depleted uranium oxide impact tests, would cause the release of more than 0.5% of the material as particulates with diameters less than 100 microns. For the 2-assembly package, this would be equivalent to a release of 225 grams, well in
313
excess of 100 A2. Only if the assembly structural materials and fuel rod cladding provide a great deal of impact resistance will a MOX assembly be able to meet the LDM impact test.
Another large source of uncertainty comes from the LDM qualification test itself. The feasibility and potential environmental consequences of carrying out these tests on actual MOX fuel have not been seriously considered. It is possible that the industry will resort to demonstrating LDM with simulant materials or relying entirely on computer modeling. Neither of these methods will provide assurance that the behavior of actual MOX pellets is being accurately represented. France's Institute of Nuclear Safety and Protection (IPSN) recently voiced its concern about the feasibility and reproducibility of LDM qualification tests. [21]
Nonetheless, BNFL has indicated that it is moving ahead on qualification of U-MOX as LDM, and that "together with other European nuclear companies, [BNFL] has embarked on a full development of an LDM test facility in line with the requirements of the published LDM regulations." [22]
If the industry finds a way to qualify U-MOX as LDM, clearing the way for large-scale U-MOX air shipments in Type B casks, the consequences could be disastrous. For a crash at the not inconceivable speed of 140 m/s, it is unreasonable to assume that a Type B cask would retain any containment ability whatsoever. Such an impact could cause a dispersible release on the order of 1% for particles smaller than 10 microns (corresponding to 450 grams of plutonium per cask) and 10% for particles smaller than 100 microns (corresponding to 4.5 kg of plutonium per cask). A typical cargo could consist of several casks, all of which must be presumed to fail under such severe conditions. These releases are enormous from a radiological perspective. Dose rates at ten miles from the crash site could well exceed 50 milliSievert per hour and could result in as many as tens of thousands of latent cancers if the crash were to occur in a densely populated area. The crash and prolonged fire of an El Al jet at an apartment complex in Amsterdam in 1992 serves as a stark reminder that this type of accident is very much in the realm of possibility.
314
End Notes
1. International Atomic Energy Agency, "The Air Transport of Radioactive Material in Large Quantifies or With High Activity," IAEA-TECDOC-702, April 1993.
2. Even attempts to design casks to withstand more severe accident conditions than the Type B test have not been successful; for example, when Japan in 1987 tested a cask specifically developed to meet the stricter US domestic air transport standards, it failed to survive the impact test [P. Leventhal, M. Hoenig and A. Kuperman, "Air Transport of Plutonium Obtained by the Japanese from Nuclear Fuel Controlled by the United States," March 3, 1987, p. 5].
3. Westinghouse Electric Corporation, "Plutonium Disposition in Existing Pressurized Water Reactors," report prepared for the U.S. Department of Energy, DOE/ SF/19683--6, June 1, 1994, p. 2.7-2.6.
4. J. Charles and 0. Konirsch, "Transportation by Road of Plutonium as a Reusable Product" Proceedings of the 11th International Conference on the Packaging and Transportation of Radioactive Materials (PATRAM '95) (December 3-8, 1995, Las Vegas, Nevada), p. 777.
5. Science and Technology Agency of Japan, "Application for Design Change Approval of Nuclear Fuel Transport Cask, FS-47 (in Japanese).
6. COGEMA, "Le Retour Au Japon Du Plutonium," 1992.
7. Alan Hughes, BNFL Public Affairs Division, letter to Fred Barker, 24 January 1997.
8. Ibid.
9. F. Henry and C. Williamson, "Rigid Polyurethane Foam for Impact and Thermal Protection," Proceedings of the 11th International PATRAM Conference, December 3-8, 1995, Las Vegas, Nevada, p. 1161.
10. Ibid.
11. T. Sanders et al., "A 'Method for Determining the Spent-Fuel Contribution to Transport Cask Containment Requirements," Sandia Report SAND90-2406, Sandia National Laboratories, November 1992, p. IV-23.
12. lbid, p. III-6.
13. Ibid.
14. Ibid, p. IV-12 --IV-16.
15. ECO Engineering Inc (Annapolis, Maryland, USA), "A Review of the Proposed Marine Transport of Reprocessed Plutonium from Europe to Japan, March 1992; E. Lyman, "Safety Issues in the Sea Transport of Vitrified High-Level Radioactive Wastes to Japan," Center for Energy and Environmental Studies, Princeton University, prepared for the Nuclear Control Institute, Greenpeace International and CNIC Tokyo, December 1994.
16. See discussion in E. Lyman, "Addressing Safety Issues in the Sea Transport of Radioactive Materials," presentation to the International Maritime Organization (IMO) Special Consultative Meeting, 4-6 March 1996, London, p. 8.
17. Alan Hughes, BNFL Public Affairs Division, op cit.
18. F. Lange, F. Nitsche, F-W. Collin and M. Cosack, "Requirements for Very Low Dispersible Material (VLDM), TC-946, Working Paper No. 11, IAEA Technical Committee Meeting, Vienna, 15-19 May 1995.
19. F. Lange and F. Nitsche "Contribution to Technical Committee Meeting," Working Paper 8, IAEA, Vienna, 29 August - 2 September 1994.
20. H. Seehars and D. Hochrainer, 'Durchffihrung von Experimenten zur Unterstutzung der Annahmen zur Freisetzung von Plutonium bei einem Flugzeugabsturz," (in German), Fraunhofer-Institute, SR 0205A, March 1982.
21. A. MacLachlan and G. Seneviratne, "France's ISPN Raises Concerns About New IAEA Transport Standards," Inside N.R.C., September 16,1996, p. 12.
22. Alan Hughes, BNFL Public Affairs Division, op cit.
![]() What's
New
What's
New ![]() Sea Shipment Page
Sea Shipment Page ![]() Home
Page
Home
Page